Simplify Medical Document Processing and Submission
Healthcare and life sciences (HLS) organizations face challenges with inefficient document workflows that lead to operational ineffectiveness, errors, and compliance problems
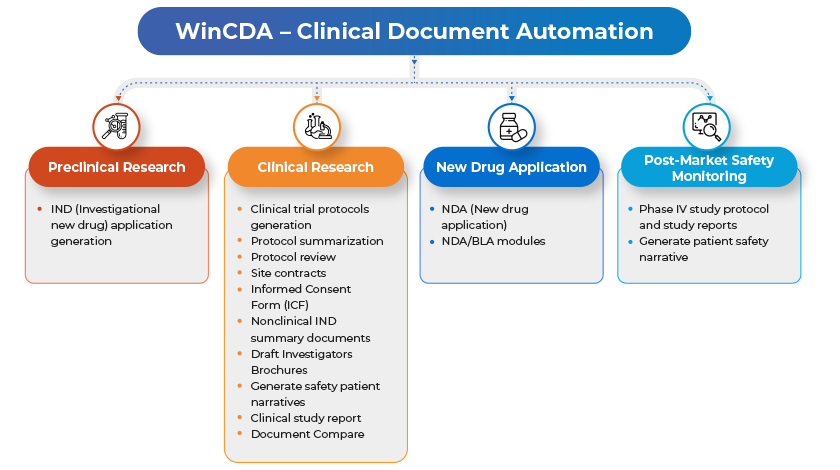
To address these issues WinWire built an intelligent clinical document automation solution that can greatly enhance the quality of medical documentation to meet regulatory standards and guidelines.