A Global Animal Health and Nutrition Leader Embraces Generative AI for Innovative Pet Care Solutions
Navigating the Future of
Generative AI
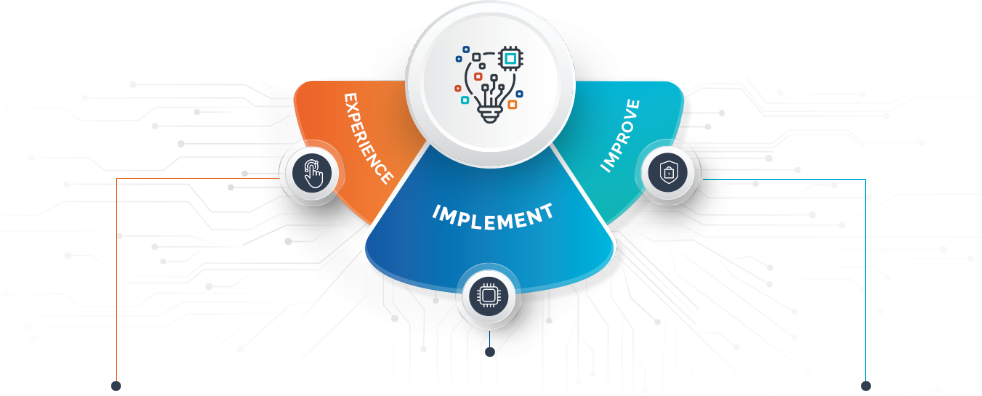
Leveraging Azure OpenAI Services for Transformative Business Growth
Start Your AI JourneyDelivering Innovative Healthcare Solutions
Helping Fortune 500 Healthcare customers innovate and transform
Learn about healthcare solutions